Coronary Angioplasty
In January 2023 Braun Kenya has started selling their AESCULAP Coronary Vascular Products in Kenya. This portfolio, that had previously been marketed by a local distributor, includes:
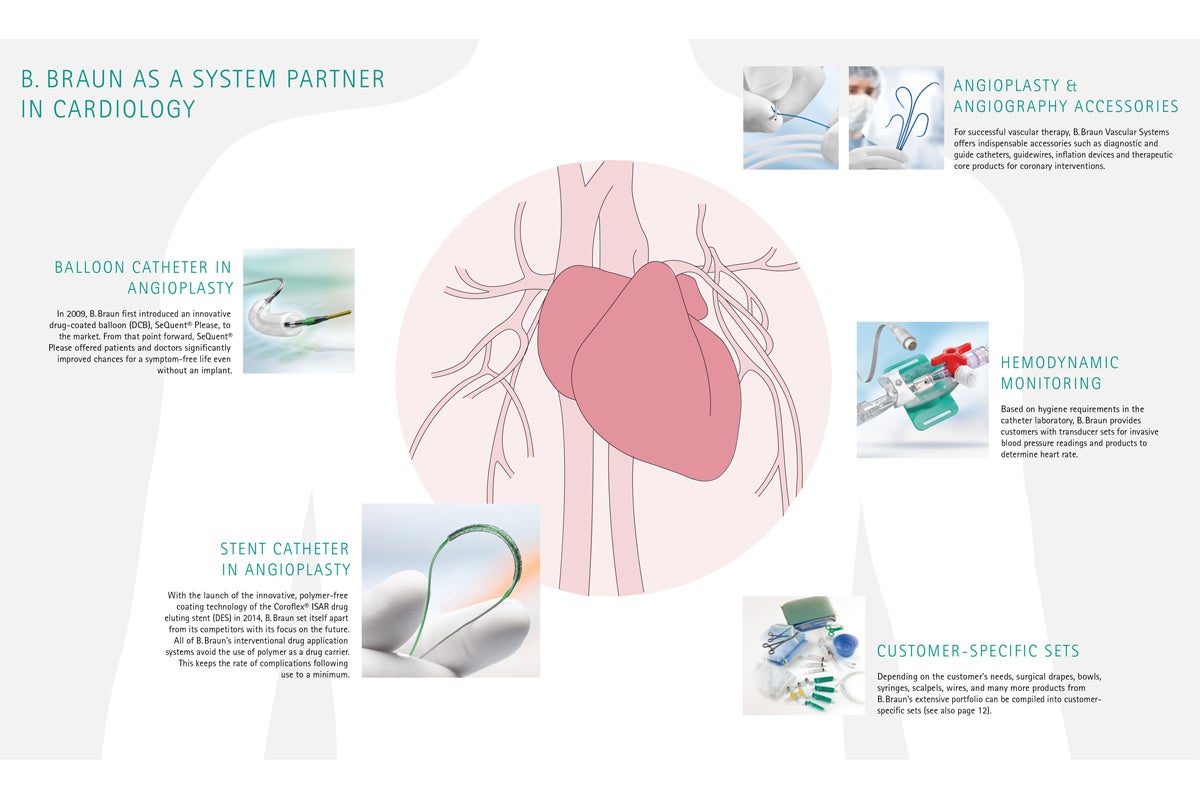
- Drug-coated Balloons (DCB) Sequent Please Neo
- PTCA Balloons (POBA) Sequent Neo, Sequent Neo NC
- Drug-eluting Stents Coroflex Isar Neo
- Diagnostic Catheters Angiodyn
- Hydrophilic guide Catheters Serpia
B. Braun Kenya is the only company offering Drug-Coated Balloons to the Kenyan market.
In recent years, B. Braun's SeQuent® Please product family has demonstrated its functionality and efficacy for coronary lesion therapy in more than 110 published studies with more than 25,000 documented patients in more than 20 countries. We are convinced that by providing this level of innovation at truly affordable prices, we can help to protect and improve the health of people in East Africa.
Affordable Innovation
B. Braun Kenya is offering this concept to all 11 Cardiac Labs currently operating in Kenya. Within the first weeks of our product launch, we have already signed consignment contracts with leading private hospitals. Once the current tender period has expired our concept of affordable innovations will be extended to the 3 Cathlabs that are serving the public sector in Kenya.
Interventional Cardiology helps to diagnose and treat coronary diseases. Let us work together to make this valuable medical discipline accessible to more patients in East Africa.
"This product provides a controlled release of the drug sirolimus in order to prevent restenosis. The coating, together with the drug, is completely absorbable within three months, which should significantly reduce the risk of later inflammatory reactions," explains Dr. Jorge Calisse, who is responsible for stent development at B. Braun Vascular Systems in Berlin. Over the years, B. Braun has established itself as one of the leading European manufacturers of products for interventional angioplasty. For up to twelve months, the patient must also take medication that keeps platelets from clumping in order to prevent dangerous blood clots from forming in the stent. If this is contraindicated, then it makes sense, depending on the disease, not to implant a stent in the first place.
System partner in cardiology
Instead, the doctor can place and inflate the drug-coated SeQuent® Please NEO balloon catheter along the vascular occlusion, another innovation by B. Braun, along the vascular occlusion. The balloon surface then releases a growth-inhibitory drug, achieving sustained vasodilatation without implanting a stent. The treatment is performed under local anesthesia and the patient can leave the hospital soon after the procedure. "It is a minimally-invasive, non-surgical technique used to dilate narrowed or blocked blood vessels mechanically," explains Dr. Fleck. The treatment is performed under local anesthesia and the patient can leave the hospital soon after the procedure.